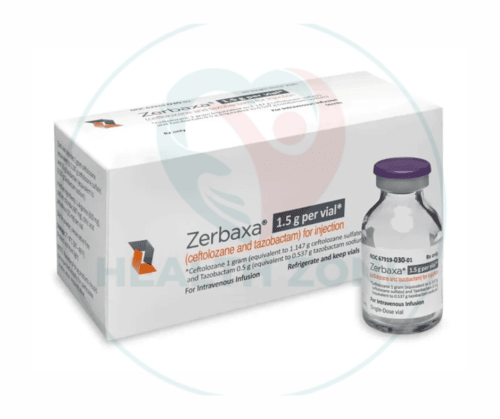
ZERBAXA® is an innovative antibiotic developed by Merck & Co., Inc., specifically designed to treat complicated infections caused by multidrug-resistant bacteria. Here, we will cover its formulation, uses, advantages, side effects, dosage, precautionary measures, year of invention, company information, and pricing in the UK and USA.
Formulation of ZERBAXA® (Ceftolozane and Tazobactam)
ZERBAXA® is a combination of two active ingredients:
- Ceftolozane: A cephalosporin antibiotic that targets bacterial cell wall synthesis.
- Tazobactam: A beta-lactamase inhibitor that helps protect ceftolozane from being broken down by certain bacteria.
Uses of ZERBAXA® (Ceftolozane and Tazobactam)
ZERBAXA® is primarily indicated for:
- Complicated Urinary Tract Infections (cUTIs): Including pyelonephritis.
- Complicated Intra-Abdominal Infections (cIAIs): When used in combination with metronidazole.
It is particularly effective against gram-negative bacteria, including Pseudomonas aeruginosa, which are often resistant to other antibiotics.
Advantages of ZERBAXA® (Ceftolozane and Tazobactam)
- Broad Spectrum of Activity: ZERBAXA® is effective against a wide range of multidrug-resistant organisms, making it a critical option in difficult-to-treat infections.
- Combination Therapy: The dual action of ceftolozane and tazobactam provides enhanced efficacy against resistant bacterial strains.
- Reduced Risk of Resistance: By combining two different mechanisms of action, ZERBAXA® helps reduce the likelihood of developing antibiotic resistance.
Side Effects of ZERBAXA® (Ceftolozane and Tazobactam)
While ZERBAXA® is generally well-tolerated, some patients may experience side effects, including:
- Nausea
- Diarrhea
- Headache
- Elevated liver enzymes
Dosage of ZERBAXA® (Ceftolozane and Tazobactam)
The typical dosing regimen for ZERBAXA® is:
- Adults: The recommended dose is 1.5 grams (1 gram of ceftolozane and 0.5 grams of tazobactam) administered intravenously every 8 hours for a duration of 4 to 14 days, depending on the type and severity of the infection.
Precautionary Measures of ZERBAXA® (Ceftolozane and Tazobactam)
Before starting treatment with ZERBAXA®, healthcare providers should consider:
- Renal Function: Regular monitoring of renal function is crucial, as dosage adjustments may be required for patients with impaired kidney function.
- Allergic Reactions: Assess for any history of allergies to penicillins or cephalosporins, as cross-reactivity can occur.
- Co-administration with Other Drugs: Review potential drug interactions with other medications the patient may be taking.
Year of Invention of ZERBAXA® (Ceftolozane and Tazobactam)
ZERBAXA® was approved by the U.S. Food and Drug Administration (FDA) in December 2014, marking a significant advancement in the treatment of complicated bacterial infections.
Company Information of ZERBAXA® (Ceftolozane and Tazobactam)
Merck & Co., Inc., also known as MSD outside the USA and Canada, is a leading global healthcare company focused on research and innovation. Founded in 1891, Merck is dedicated to improving health worldwide through its extensive portfolio of pharmaceuticals and vaccines. ZERBAXA® reflects their commitment to addressing the urgent need for effective antibiotics in the face of rising antibiotic resistance.
Price in USA and UK of ZERBAXA® (Ceftolozane and Tazobactam)
The cost of ZERBAXA® can vary based on healthcare plans and regional pricing. In the USA, the estimated price for a course of treatment can range from $1,800 to $2,500. In the UK, the NHS pricing typically ranges from £800 to £1,200 for a treatment course, depending on the specific healthcare provider and circumstances.
Conclusion
ZERBAXA® (ceftolozane and tazobactam) offers a powerful option for treating complicated infections caused by multidrug-resistant bacteria. Its unique formulation and dual-action mechanism make it a vital tool in the fight against antibiotic resistance. As always, consult a healthcare provider for personalized advice and treatment options related to antibiotic use.