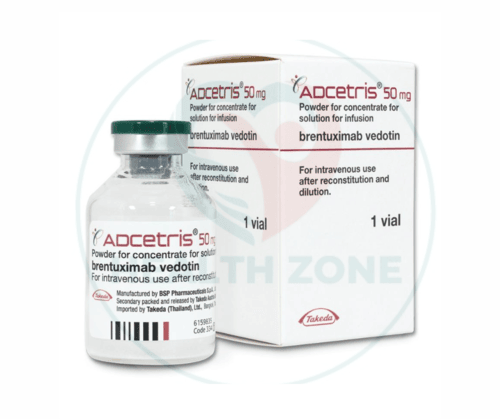
ADCETRIS (brentuximab vedotin) is an innovative medication developed by Pfizer, primarily used in the treatment of specific types of lymphomas, including Hodgkin lymphoma and systemic anaplastic large cell lymphoma (sALCL). This drug is a targeted therapy that combines an antibody with a chemotherapy agent, offering a novel approach to cancer treatment.Here we provide a detailed examination of ADCETRIS, covering its formulation, uses, advantages, side effects, dosage, precautionary measures, historical context, and pricing in the UK and US.
Formulation of ADCETRIS
ADCETRIS, a targeted therapy used in the treatment of certain types of lymphomas, is specifically formulated for intravenous administration. Its formulation ensures precision in dosing and delivery to maximize therapeutic efficacy while minimizing side effects. Below is the detailed formulation and preparation information:
Injectable Solution:
- Concentration: ADCETRIS is supplied as a lyophilized powder for reconstitution. The vial contains 50 mg of Brentuximab Vedotin per vial, which, when reconstituted, results in a solution with a concentration of 5 mg/mL.
- Packaging: Each vial is single-use and sterile, ensuring optimal safety for clinical use.
- Storage Conditions: The vials should be stored under refrigeration at 2°C to 8°C (36°F to 46°F) and protected from light. Avoid freezing the product.
Uses of ADCETRIS
ADCETRIS is indicated for:
- Hodgkin Lymphoma: Used for patients who have relapsed after autologous stem cell transplant or who have failed other therapies.
- Systemic Anaplastic Large Cell Lymphoma: Effective in treating this aggressive form of lymphoma, particularly in patients who have received at least one prior therapy.
- Brentuximab Vedotin Combination Therapy: Can be used in combination with chemotherapy agents for enhanced efficacy in certain treatment regimens.
Advantages of ADCETRIS
ADCETRIS offers several significant advantages:
- Targeted Therapy: Its design allows for the delivery of chemotherapy directly to cancer cells, potentially reducing damage to healthy tissue.
- Improved Outcomes: Clinical studies have shown it can lead to better response rates and overall survival in patients with relapsed or refractory lymphomas.
- Well-Characterized Mechanism: As an antibody-drug conjugate, it combines specificity with potency, making it effective in targeting malignant cells.
Side Effects of ADCETRIS
While ADCETRIS is generally well-tolerated, it can cause side effects, including:
- Common Side Effects: Nausea, fatigue, vomiting, diarrhea, and neutropenia (low white blood cell count).
- Serious Side Effects: Peripheral neuropathy, infections, and potential liver toxicity. Regular monitoring is essential to manage these risks.
- Allergic Reactions: Rarely, patients may experience hypersensitivity reactions requiring immediate medical attention.
Dosage of ADCETRIS
The dosage of ADCETRIS typically depends on the patient’s body surface area:
- Initial Dose: Commonly administered at 1.8 mg/kg intravenously every three weeks.
- Adjustment: Doses may be adjusted based on tolerability and side effects. It’s crucial for healthcare providers to tailor treatment plans to individual patient needs.
Precautionary Measures of ADCETRIS
Patients considering ADCETRIS should be aware of the following precautions:
- Infection Risk: Due to potential immunosuppression, patients should be monitored for signs of infection and may require prophylactic antibiotics.
- Neuropathy Monitoring: Regular assessment for signs of peripheral neuropathy is necessary, as this can affect daily functioning.
- Pregnancy and Breastfeeding: ADCETRIS is contraindicated in pregnancy and should not be used during breastfeeding due to potential risks to the fetus or infant.
Year of Invention & Company Information
ADCETRIS was first approved by the U.S. Food and Drug Administration (FDA) in 2011. Developed by Seattle Genetics, it was later acquired by Pfizer, a leading global pharmaceutical company known for its commitment to advancing healthcare through innovative therapies and extensive research.
Price of ADCETRIS in UK & US
The cost of ADCETRIS can vary based on several factors:
- United States: The average price is approximately $20,000 per dose, though this can differ based on insurance coverage and specific treatment plans.
- United Kingdom: The cost typically ranges from £10,000 to £15,000 per cycle, again depending on the treatment protocol and healthcare provider.
Conclusion
ADCETRIS represents a significant advancement in the treatment of specific lymphomas, offering hope to patients facing challenging diagnoses. Its targeted mechanism, coupled with the ability to improve patient outcomes, underscores the importance of continued innovation in oncology. As with all medications, thorough consultation with healthcare professionals is essential to ensure safe and effective treatment tailored to individual needs.